
A citywide prevention program using ivermectin as prophylaxis for COVID-19 was implemented in Itajaí, a southern city in Brazil in the state of Santa Catarina between July 2020 and December 2020.
In early July, the city mayor of Itajaí, Dr. Volnei José Morastoni announced a citywide use of Ivermectin against COVID-19. The mayor distributed Ivermectin kits totaling 1.5 million tablets to the residents of Itajaí. The study of this program was published on NIH website.
According to this comprehensive study, the regular use of ivermectin as a prophylactic agent was associated with significantly reduced COVID-19 infection, hospitalization, and death rates. The ivermectin non-users were two times more likely to die of COVID-19 than ivermectin users in the overall population analysis.
Read the summary of the study:
Materials and methods: We analyzed data from a prospective, observational study of the citywide COVID-19 prevention with ivermectin program, which was conducted between July 2020 and December 2020 in Itajaí, Brazil. Study design, institutional review board approval, and analysis of registry data occurred after completion of the program. The program consisted of inviting the entire population of Itajaí to a medical visit to enroll in the program and to compile baseline, personal, demographic, and medical information. In the absence of contraindications, ivermectin was offered as an optional treatment to be taken for two consecutive days every 15 days at a dose of 0.2 mg/kg/day. In cases where a participating citizen of Itajaí became ill with COVID-19, they were recommended not to use ivermectin or any other medication in early outpatient treatment. Clinical outcomes of infection, hospitalization, and death were automatically reported and entered into the registry in real time. Study analysis consisted of comparing ivermectin users with non-users using cohorts of infected patients propensity score-matched by age, sex, and comorbidities. COVID-19 infection and mortality rates were analyzed with and without the use of propensity score matching (PSM).
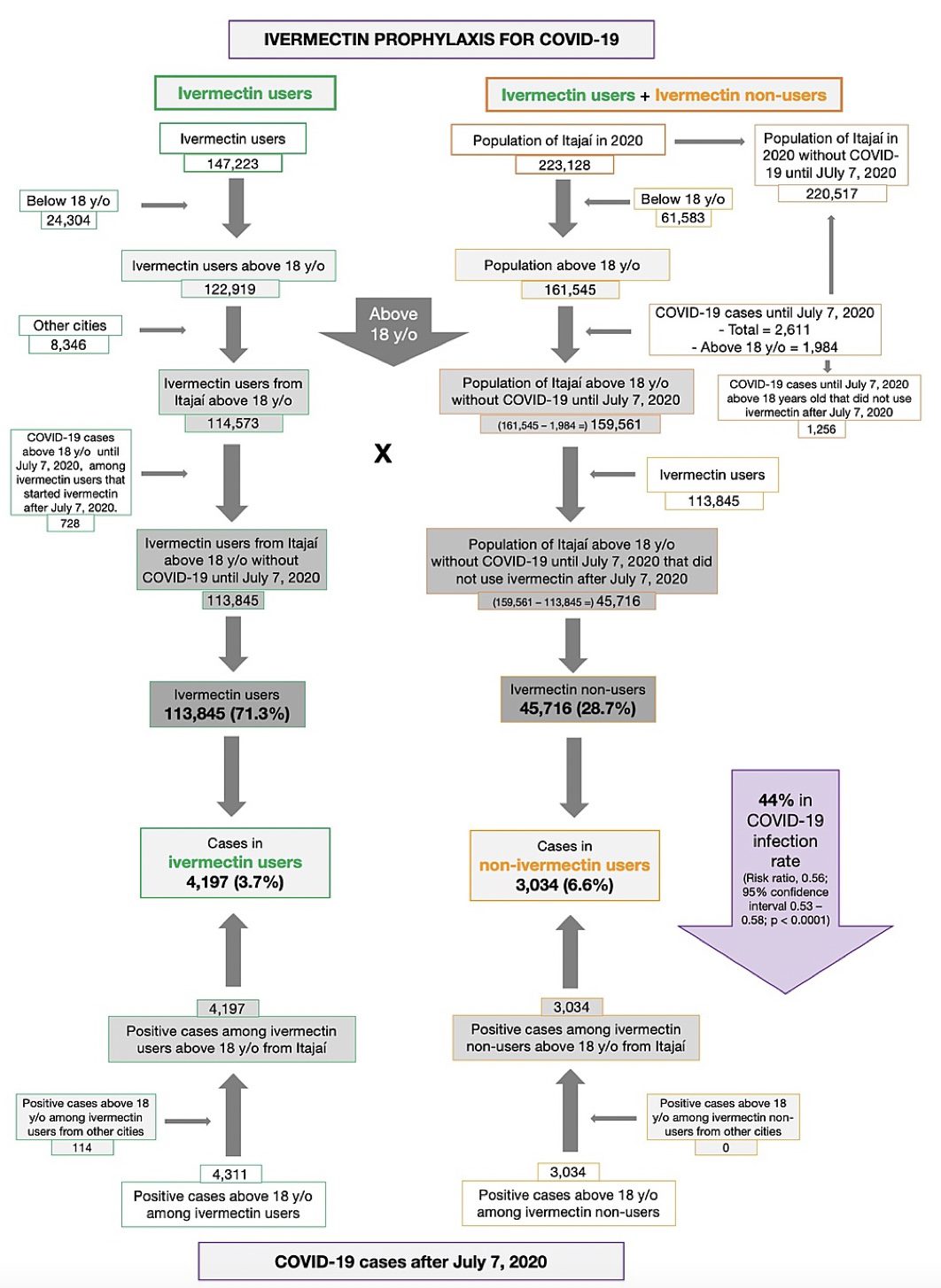
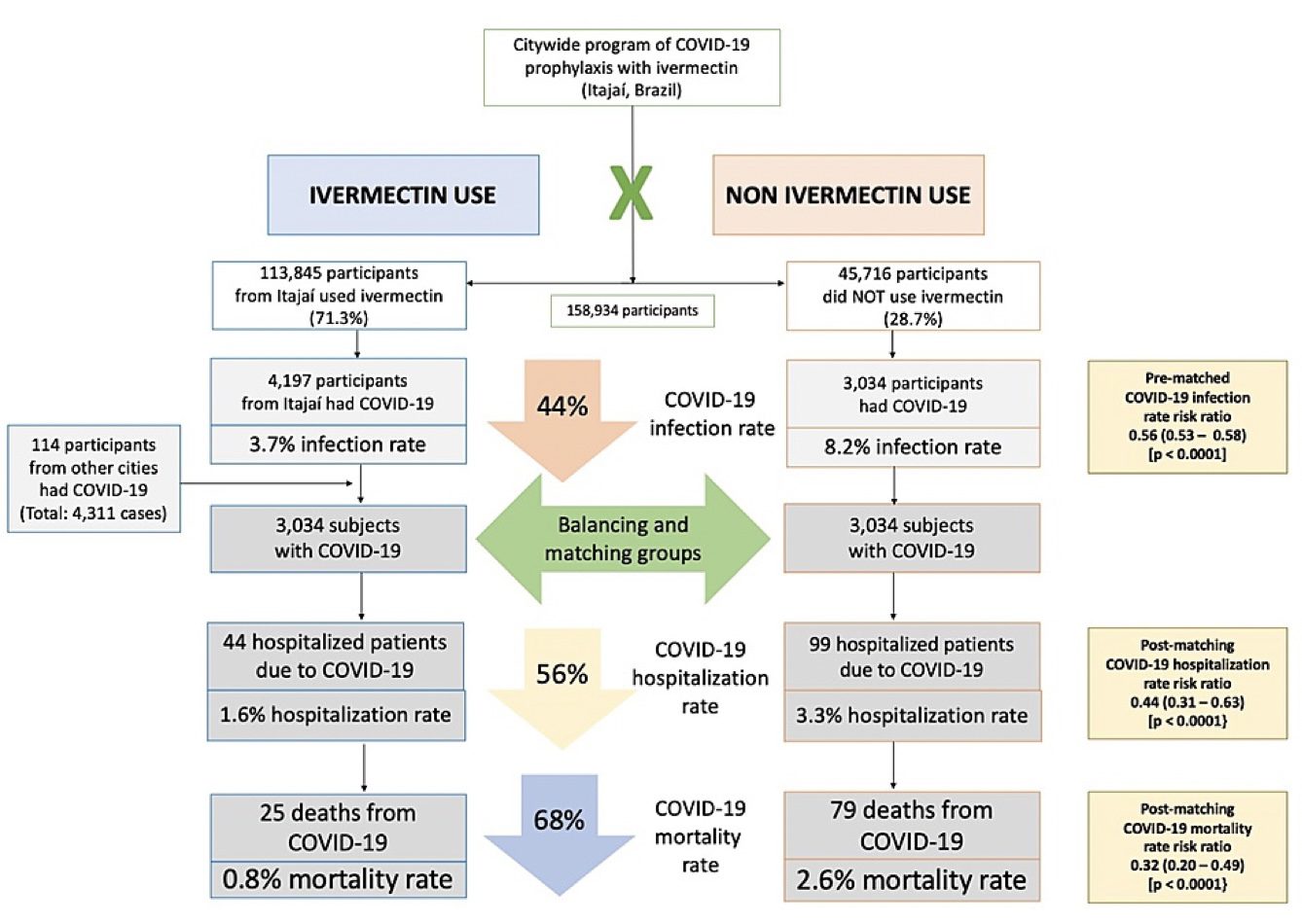
Results: Of the 223,128 citizens of Itajaí considered for the study, a total of 159,561 subjects were included in the analysis: 113,845 (71.3% of the population above 18 years old) regular ivermectin users and 45,716 (23.3%) non-users. Of these, 4,311 ivermectin users were infected, among which 4,197 were from the city of Itajaí (3.7% infection rate), and 3,034 non-users (from Itajaí) were infected (6.6% infection rate), with a 44% reduction in COVID-19 infection rate (risk ratio [RR], 0.56; 95% confidence interval (95% CI), 0.53-0.58; p < 0.0001). Using PSM, two cohorts of 3,034 subjects suffering from COVID-19 infection were compared. The regular use of ivermectin led to a 68% reduction in COVID-19 mortality (25 [0.8%] versus 79 [2.6%] among ivermectin non-users; RR, 0.32; 95% CI, 0.20-0.49; p < 0.0001). When adjusted for residual variables, reduction in mortality rate was 70% (RR, 0.30; 95% CI, 0.19-0.46; p < 0.0001). There was a 56% reduction in hospitalization rate (44 versus 99 hospitalizations among ivermectin users and non-users, respectively; RR, 0.44; 95% CI, 0.31-0.63; p < 0.0001). After adjustment for residual variables, reduction in hospitalization rate was 67% (RR, 0.33; 95% CI, 023-0.66; p < 0.0001).
Of the 113,845 prophylaxed subjects from the city of Itajaí, 4,197 had a positive RT-PCR SARS-CoV-2 (3.7% infection rate), while 3,034 of the 37,027 untreated subjects had positive RT-PCR SARS-CoV-2 (6.6% infection rate), a 44% reduction in COVID-19 infection rate (risk ratio [RR], 0.56; 95% confidence interval (95% CI), 0.53-0.58; p < 0.0001). An addition of 114 subjects who used ivermectin and were infected were originally from other cities but were registered as part of the program, in a total of 4,311 positive cases among ivermectin users. For the present analysis, the 4,311 positive cases among subjects that used ivermectin and 3,034 cases among subjects that did not use ivermectin were considered. After PSM, two cohorts of 3,034 subjects were created.
Baseline characteristics of the 7,345 subjects included prior to PSM and the baseline characteristics of the 6,068 subjects in the matched groups are shown in Table 1. Prior to PSM, ivermectin users had a higher percentage of subjects over 50 years old (p < 0.0001), higher prevalence of T2D (p = 0.0004), hypertension (p < 0.0001), and CVD (p = 0.03), and a higher percentage of Caucasians (p = 0.004), than non-users. After PSM, all baseline parameters were similar between groups. Figure 2 summarizes the main findings of this study.
Hospitalization and mortality rates in ivermectin users and non-users in propensity score-matched analysis
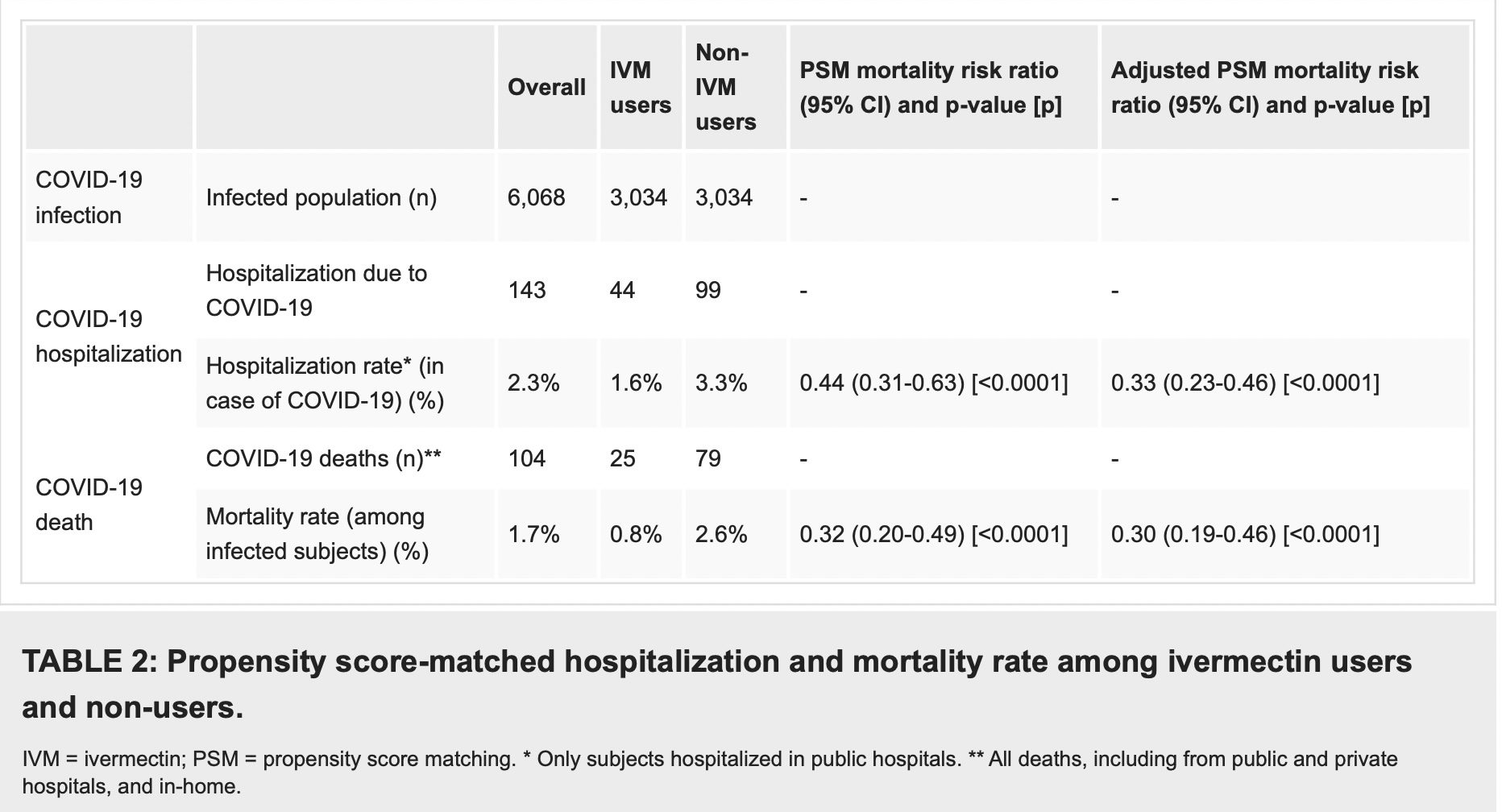
As described in Table 2, after employing PSM, of the 6,068 subjects (3,034 in each group), there were 44 hospitalizations among ivermectin users (1.6% hospitalization rate) and 99 hospitalizations (3.3% hospitalization rate) among ivermectin non-users, a 56% reduction in hospitalization rate (RR, 0.44; 95% CI, 0.31-0.63). When adjustment for variables was employed, the reduction in hospitalization rate was 67% (RR, 0.33; 95% CI, 023-0.66; p < 0.0001).
There were 25 deaths among ivermectin users (0.8% mortality rate) and 79 deaths among non-ivermectin users (2.6% mortality rate), a 68% reduction in mortality rate (RR, 0.32; 95% CI, 0.20-0.49). When PSM was adjusted, reduction in mortality rate was 70% (RR, 0.30; 95% CI, 0.19-0.46; p < 0.0001).
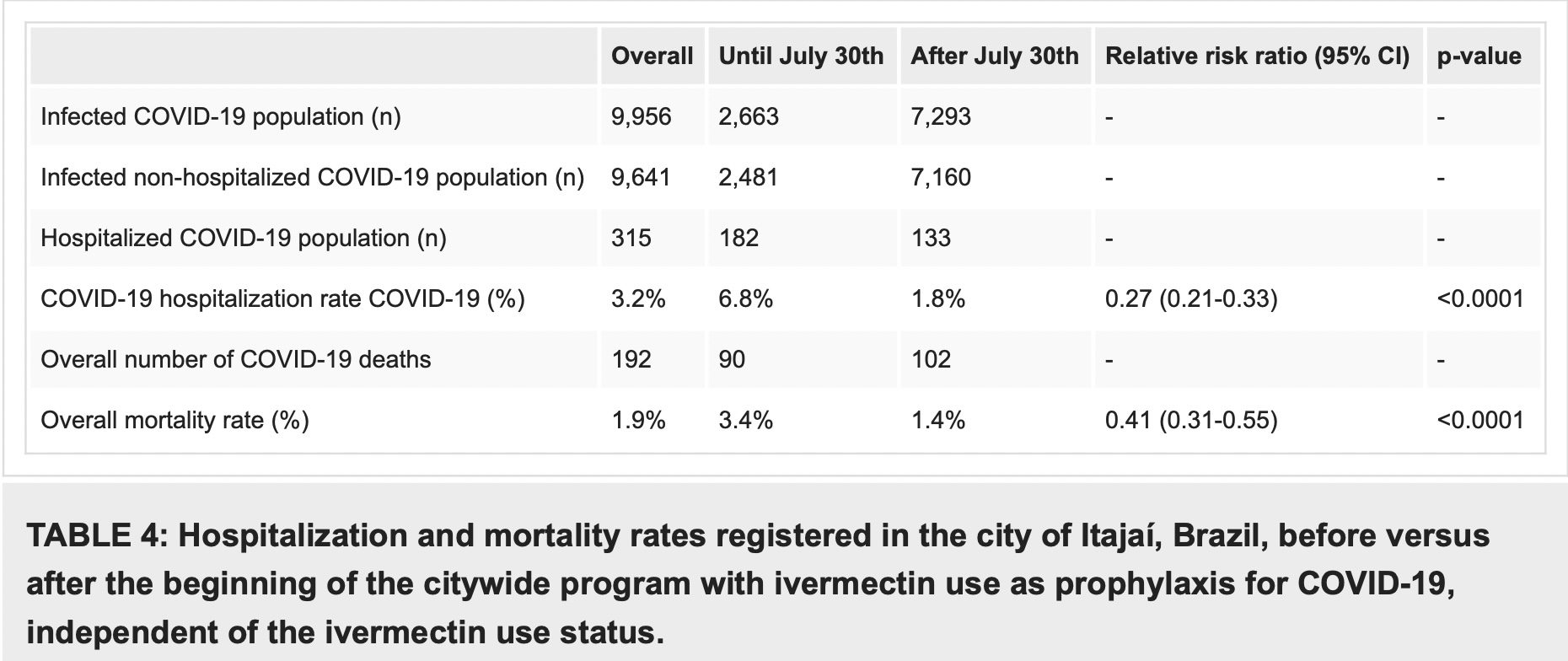
In a comparison of citywide COVID-19 hospitalization rates prior to and during the program, COVID-19 mortality decreased from 6.8% before the program with prophylactic use of ivermectin, to 1.8% after its beginning (RR, 0.27; 95% CI, 0.21-0.33; p < 0.0001), and in COVID-19 mortality rate, from 3.4% to 1.4% (RR, 0.41; 95% CI, 0.31-0.55; p < 0.0001) (Table 4).
Final discussion: In this citywide ivermectin prophylaxis program, a large, statistically significant decrease in mortality rate was observed after the program began among the entire population of city residents. When comparing subjects that used ivermectin regularly, non-users were two times more likely to die from COVID-19 while ivermectin users were 7% less likely to be infected with SARS-CoV-2 (p = 0.003).
Although this study is not a randomized, double-blind, placebo-controlled clinical trial, the data were prospectively collected and resulted in a massive study sample that allowed adjustment for numerous confounding factors, thus strengthening the findings of the present study.
Due to the well-established, long-term safety profile of ivermectin, with rare adverse effects, the absence of proven therapeutic options to prevent death caused by COVID-19, and lack of effectiveness of vaccines in real-life all-cause mortality analyses to date, we recommend that ivermectin be considered as a preventive strategy, in particular for those at a higher risk of complications from COVID-19 or at higher risk of contracting the illness, not as a substitute for COVID-19 vaccines, but as an additional tool, particularly during periods of high transmission rates.
Cite this article as: Kerr L, Cadegiani F A, Baldi F, et al. (January 15, 2022) Ivermectin Prophylaxis Used for COVID-19: A Citywide, Prospective, Observational Study of 223,128 Subjects Using Propensity Score Matching
Read the full study here:
Ivermectin Prophylaxis Used for COVID-19: A Citywide, Prospective, Observational Study of 223,128 Subjects… by Jim Hoft on Scribd



No comments:
Post a Comment